Analytical Services for Advanced Therapies
Our analytical services for advanced therapies enable you to successfully address the needs of your cell and gene therapy, ensuring your product meets the highest quality standards. Our experienced team of scientists excel in the development of custom analytical methods matched to the unique demands of your product profile.

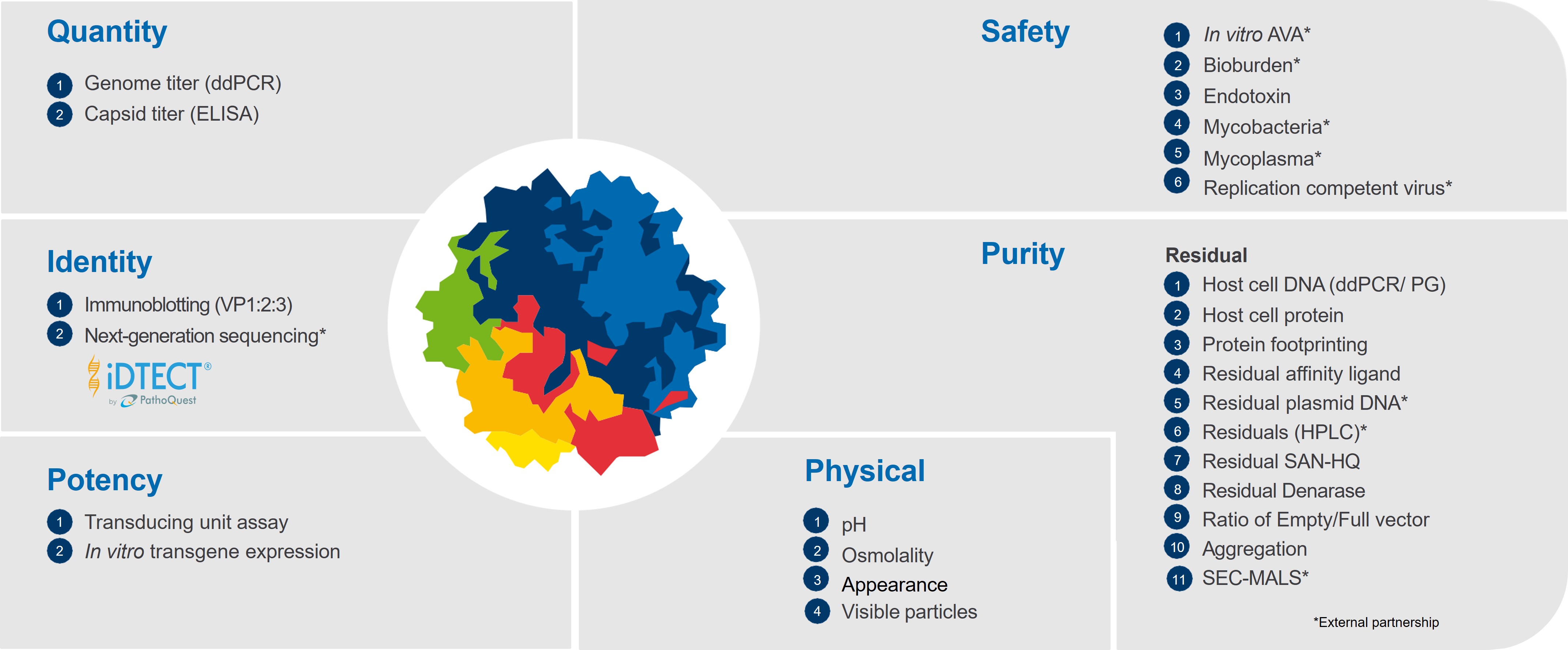
Comprehensive Analytical Portfolio for Advanced Therapies

Analytical testing is critical to understanding your gene therapy product profile from preclinical to commercial, ensuring it is safe, effective and commercially viable. At Rentschler Biopharma we offer a comprehensive suite of cutting-edge analytical technologies in-house for testing and characterization of your advanced therapies. Our analytical services span throughout the product lifecycle from process development to clinical and commercial cGMP release testing.
Our analytical experts specialize in the development, tech transfer, qualification and validation of methods to guarantee high product quality and consistency during manufacturing. We collaborate with you at every step to ensure that the developed methods align with the specific needs of your viral vector product. Our team secures a seamless transfer of analytical methods to our quality control department or trusted partner Contract Research Organizations (CROs), providing effective qualification, validation, and release testing.
Explore our Analytical Toolbox Technologies
Developing analytical methods and testing complex advanced therapies requires expertise and state-of-the-art equipment. Benefit from our customized capabilities designed to meet your specific requirements and timelines.
Quality Control and Stability Services
At our dedicated advanced therapy CDMO facility in Stevenage, UK, we provide raw material, drug substance and drug product release testing, as well as stability studies to guarantee the highest quality and safety of your gene therapies. Our QC team utilizes innovative analytical technologies to ensure successful cGMP manufacturing operations and compliance with regulatory requirements. Our comprehensive QC services include:
- Raw materials
- In-process control and intermediates testing
- Drug substance and drug product release testing
- QP certification for each batch
- Stability testing

Highlights
Get in Touch with our Business Development Team
Are you interested in learning more? We look forward to hearing from you!
Robert Panting
General Manager, ATMP
